Presentation Title: High Performance When You Least Expect It: How Halide Perovskites Are Transforming Photovoltaics
Prof. Mercouri Kanatzidis presented this talk in the webinar on Materials Science, Engineering and Technology organized by Vebleo
Affiliation: Department of Chemistry, Northwestern University, Evanston, Illinois 60208, USA
Biography
Mercouri Kanatzidis was born in Thessaloniki, Greece in 1957. After obtaining a BSc degree from Aristotle University in Greece, he received his PhD degree in Chemistry from the University of Iowa in 1984.
Prof. Mercouri Kanatzidis was a postdoctoral research associate at the University of Michigan and Northwestern University and is currently Charles E. and Emma H. Morrison Professor of Chemistry at Northwestern University.
Prof. Mercouri Kanatzidis’s research areas include halide perovskites, solid state and coordination chemistry of chalcogenide compounds, design and synthesis of new materials, solar energy materials, thermoelectric materials, intermetallics, porous semiconductors and quantum materials.
Prof. Mercouri Kanatzidis is the recipient of the Royal Chemical Society DeGennes Prize 2015; 2015 ENI Award for the ‘‘Renewable Energy Prize’’ category; the ACS Award in Inorganic Chemistry 2016; and the American Physical Society 2016 James C. McGroddy Prize for New Materials; 2016 Samson Prime Minister’s Prize for Innovation in Alternative Fuels for Transportation; 2019 – DOE Ten at Ten Scientific Ideas Award for the first demonstration of all-solid-state solar cells using halide perovskite materials.
Abstract
How did the ongoing revolution in the science of halide perovskites begin? The chemical versatility and structure diversity in the class of hybrid organic inorganic main metal halides is astounding. The interplay of weak covalent and ionic bonding in the inorganic framework allows the formation of an amazingly broad variety of structures most of which can be divided into two larger classes: three-dimensional (3D) and 2D perovskites.
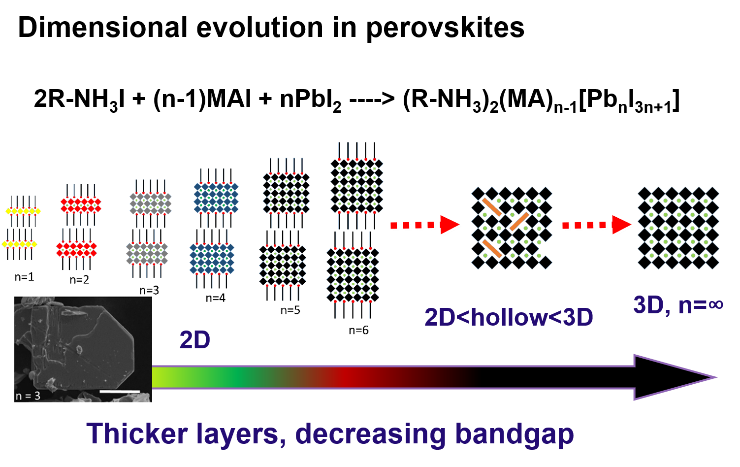
The lone pair of electrons in Pb2+ and Sn2+ impart defect tolerance beneficial to charge transport. The 2D metal halide perovskites have also become highly promising semiconductors for tunable optoelectronic devices. They have a general formula of (A’)2(A)n-1MnX3n+1, where A = Cs+, CH3NH3+ (MA), HC(NH2)2+ (FA), M = Ge2+, Sn2+, Pb2+ and X = Cl–, Br–, I–, are the perovskite components and A’+ = RNH3 is an organic spacer.
There are three kinds of 2D organic inorganic hybrid perovskites so far: Ruddlesden-Popper, Cation-ordered and Jacobson-Dion. These vary from one another in ways the inorganic slabs stack and the way the spacer cations interact with the inorganic slabs. Generally, 2D perovskites form from solution via the bottom-up self-assembly of individual, semiconducting perovskite sheets having an adjustable slab thickness of up to few nanometers, separated by insulating bulky organic molecules.
As a result, they behave as natural multiple quantum wells (QWs) with the semiconducting perovskite layers representing the wells and the insulating organic spacers representing the barriers. The width of the barrier is fixed and depends only on the length of the A’ cation, while the width of the well can be adjusted by varying the thickness of perovskite slabs, which is defined by the n variable in (A’)2(A)n-1MnX3n+1.
The chemical and structural aspects of these materials will be presented and devices made from them will be described.
Graphical Abstract
This talk was delivered in the webinar organized by Vebleo